What is Essure Birth Control?
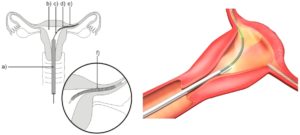
Essure was marketed as a permanent, less-invasive surgical sterilization procedure for women developed by Conceptus Inc., a subsidiary of Bayer AG. With Essure, a metallic coil designed to induce fibrosis is embedded into a woman’s Fallopian tubes to block the tubes and prevent fertilization. It was approved for use and sold in the United States starting on November 4, 2002.
Theoretically, once in place, the stainless steel and metal alloy Essure device is designed to elicit tissue growth in and around the insert over a period of three months, which eventually forms a blockage in the Fallopian tubes. The scar tissue barrier formed should prevent sperm from reaching an egg. Eventually, the body breaks down the unfertilized eggs and reabsorbs them.
However, serious complications can arise from the use of Essure, as it can dislodge and migrate inside the reproductive organs or even the stomach or the colon, causing damage to a woman’s body or a baby’s placenta. The coil can also break and cause persistent pain, bleeding, and serious, deadly infections. Essure victims have had to suffer Essure coil removal surgeries, removal of torn Fallopian tubes, hysterectomies, birth defects, and even deaths.
Currently, the Essure lawsuits in federal court has alleged negligent manufacture, negligent risk management, breach of express warranty, negligent training and fraudulent manufacture against Bayer AG. The Essure lawsuits on behalf of victims have been allowed to proceed despite Bayer’s argument of preemption.
Essure – Dangerous Complications
Women with Essure implants continue to report significant complications such as:
- Pain/Vaginal bleeding
- Nausea/Vomiting/Fainting
- Serious Infections
- Perforation or damage to reproductive organs and other organs such as the stomach or colon.
- Pregnancy – Ectopic pregnancies or pregnancy complications in the event of Essure failure
- Risks of Future medical procedure such as Essure removal, Hysterectomy or Endometrial Ablation
- Incontinence
- Bowel dysfunction
- Pelvic organ prolapse
- Sexual problems
- Changes in menstrual cycle
Terrible Consequences Suffered by Essure Victims
The Essure device is supposed to be an easy and safe way to prevent pregnancy. But that’s often not the case. Each year, the FDA receives several hundred thousand medical device reports (MDRs) of suspected device-associated deaths, serious injuries and malfunctions. The FDA uses MDRs to monitor device performance, detect potential device-related safety issues, and contribute to benefit-risk assessments of these products. The MAUDE database houses MDRs submitted to the FDA by mandatory reporters (manufacturers, importers and device user facilities) and voluntary reporters such as health care professionals, patients and consumers. Some of the MAUDE reports involving Essure contain tragic narratives.
- According to a September 2015 report, a woman found out she was six weeks pregnant after her Essure insert was implanted in 2012. Worried about the health of her baby, she consulted her doctor about the risks. She was told that the coils would “bend out of the way and they would not cause any harm to my pregnancy”. At 22 weeks she heard a loud popping sound while she was using the restroom, “like a balloon.” Water started trickling down her legs immediately afterwards. She went to the emergency room and found out that her amniotic fluid was leaking. While in the ER, the doctors attempted to stitch her cervix closed, which is known as a cerclage procedure, to try to save the pregnancy. The patient stated in the report that her doctor stopped the cerclage procedure when, shockingly, an Essure coil was found inside of her vaginal tract. Apparently, the coil was never properly implanted in the Fallopian tube and was floating around inside of her uterus during her entire pregnancy. She had to deliver her baby on her own because she was too far along in the pregnancy. She delivered a baby, whom she and her husband named Daphne, and held her in their arms. Daphne died 15 minutes later. “I had to bury my baby because of a coil,” the patient stated in the report. “I now suffer from PTSD because of the traumatizing nature in which this all happened.
- Lisa Saenz endured cramps, chronic discomfort, and menstrual periods that lasted for as long as 15 days. She later discovered one of the Essure coils had perforated her uterus.
- Janie Garcia had an Essure device implanted, and then bled uncontrollably for six months. She tried to get the device removed, but a chunk of the metal and surgical clips were left inside her. The problem had to be resolved by removing the 31-year-old’s uterus.
- Cecilia Bogle’s Essure coils broke into pieces when doctors tried to remove them after they perforated her uterus and migrated into her stomach. She now has about five and a half centimeters of metal still in her body.
Essure Removal Can Involve Complete Hysterectomies
Many women have had serious, life threatening complications with Essure. Some women’s bodies react poorly to the device. Some report autoimmune problems, migraines, hair loss, perforation of the Fallopian tubes or other organs and other issues. In these cases, a regular Essure reversal is not possible because the Fallopian tubes and uterus may be too damaged. Surgeons then opt to do a complete hysterectomy.
The long-term effects of hysterectomies can affect a woman’s quality of life. For instance, removing the uterus can lead to incontinence, bowel dysfunction, pelvic organ prolapse, sexual problems and the formation of scar tissue in the pelvic organs. Some women receive trans-vaginal mesh to treat incontinence and prolapse, an implant that may also cause its own complications like organ perforation and severe pelvic pain.
Review of Reported Problems
Many of the incidents and complications remain ignored by Bayer or the FDA. If the patient or her physician doesn’t provide the actual coil for inspection, the cases are considered inconclusive. “It is not possible to determine if a device malfunctioned if the device is unavailable for inspection,” said DiFlumeri, the Bayer representative.
The FDA has received thousands of complaints from women who suffered serious complications after receiving the non-surgical permanent birth control device Essure®, manufactured by Bayer. Some claim that Bayer knew about the adverse side effects, but concealed them from the public.
There’s a push to ban the device. Rep. Mike Fitzpatrick, D-Penn., is leading a ban on Essure called the “E-Free Act.” The bill, introduced in November, would require the FDA to withdraw its approval for Essure. On Dec. 10, Fitzpatrick addressed Congress on behalf of the women harmed by Essure:
“Their stories are real, their pain is real, and that their fight is real,”
Meanwhile, the U.S. Food and Drug Administration announced it is mandating stronger warnings and more studies for Bayer’s controversial Essure Permanent Birth Control device.
FDA is requiring a new mandatory post market clinical study “to determine heightened risks for particular women.” In addition, packages of Essure will now carry a black box warning (the agency’s strongest warning) — Issued the final guidance, “Labeling for Permanent Hysteroscopically-Placed Tubal Implants Intended for Sterilization” — and a Patient Decision Checklist designed to inform women of the risks.
Draft guidance Black Box Warning:
- WARNING: Some patients implanted with the Essure System for Permanent Birth Control have experienced and/or reported adverse events, including perforation of the uterus and/or fallopian tubes, identification of inserts in the abdominal or pelvic cavity, persistent pain, and suspected allergic or hypersensitivity reactions. If the device needs to be removed to address such an adverse event, a surgical procedure will be required. This information should be shared with patients considering sterilization with the Essure System for Permanent Birth Control during discussion of the benefits and risks of the device.
- WARNING: Some patients implanted with the Essure System for Permanent Birth Control have experienced and/or reported adverse events, including perforation of the uterus and/or fallopian tubes, identification of inserts in the abdominal or pelvic cavity, persistent pain, and suspected allergic or hypersensitivity reactions. If the device needs to be removed to address such an adverse event, a surgical procedure will be required. This information should be shared with patients considering sterilization with the Essure System for Permanent Birth Control during discussion of the benefits and risks of the device.
At Oliver-Zhang Law, our experienced attorneys in Women’s Health and defective reproductive medical products and complex medical device litigation, are currently handling Essure lawsuits nationwide. We provide confidential and free consultation in order to represent you in your Essure case with the strongest available medical and factual evidence. If you or a loved one had an Essure implant and are suffering symptoms or complications, please call us to review the details of your case to see if you have a compensable injury. Our Essure attorneys fight for women’s health rights.
Essure News:
- WXYZ News, April 2015
FDA investigates permanent birth control Essure after receiving thousands of complaints
- CBS WNCN, May 2, 2016
RALEIGH, N.C. (WNCN) — The FDA is investigating a permanent form of birth control called Essure after receiving thousands of complaints from women.
Essure court ruling in California could bring more lawsuits
- Modern Health Care, August 9, 2016
A California state court has cleared the path for nearly a dozen lawsuits to proceed that alleged that pharmaceutical company Bayer’s permanent birth control device, Essure, seriously injured patients.
Congresswoman introduces bill to force FDA to take Essure off the market
- Essure Procedure Net, November 6, 2015
A U.S. Congressman introduced a bill Wednesday aimed at taking Essure permanent birth control off the market. November 4th marks the 13th anniversary of the device’s approval by the FDA.
FDA mandates new warnings, new data for Essure contraceptive device
- CNN, March 1, 2016
(CNN)The U.S. Food and Drug Administration said it will require a new “black box warning” label for Essure, an implantable permanent contraceptive device. A black box warning in the labeling of products is “designed to call attention to serious or life-threatening risks,” according to the FDA website.

